INTRODUCTION
Obesity means a physiological state in which excessive body fat has been collected, and it may extend the hazard of various diseases such as hyperlipidemia, cardiovascular disease, type 2 diabetes, and coronary heart disease. For this reason, nowadays, obesity has become a serious worldwide problem in human health.[1,2] Numerous reasons, including genetic predisposition, excessive food intake, lack of exercise, and lifestyle, have been reported to induce obesity.[2-4] Most studies demonstrated that the storage of lipid in adipocytes by adipogenesis is one of the main causes of obesity.[5-8]
There are several pharmacologic substances available as antiobesity drugs; however, they have hazardous side effects including increased blood pressure, headache, dry mouth, insomnia, and constipation,[9,10] and thus obesity has been treated in Asian countries by various produce available naturally,[11] which is an alternative strategy for effective, safe antiobesity drug.[12]
I-SLIM, an Ayurvedic proprietary reconstituted health drink from Indusviva HealthSciences Pvt.
Ltd., Bangalore, is a polyherbal formulation in the management of obesity. With its unique combination of the antiobesity herbal active blend and with the goodness of proteins, multivitamins and enzymes, I-SLIM is considered safe and efficacious in obesity management.
I-SLIM is a proprietary blend of three well-known natural ingredients: Coleus forskohlii, Salacia reticulata, and Sesamum indicum. These ingredients are known to hinder the absorption of fat at distinct amount and kinetics independently. Individually, each of these components has been shown to inhibit fat absorption with differing degrees and dynamics. It helps in preventing the negative gastrointestinal and metabolic side effects often associated with fat blockers.[13]
C. forskohlii is well-studied plant for its supportive role in the management of weight, which has been used for centuries in traditional Indian medicine system. Torrents of chemical reactions are induced by C. forskohlii in fat reduction.[14,15]
An anti-diabetic plant, S. reticulata has a special place in the classical medicine of Ayurveda, with vested regulatory action on alpha-glucosidase, an enzyme that prevents absorption of excess dietary sugar,[16] and appears to have the ability to block fat and may reduce the rate of lipid (fat) absorption after a meal.
S. indicum also exerts this activity. This superfood is a rich source of protein and many essential minerals that are believed to support healthy cardiovascular and circulatory functions.[17]
In the present study, we investigated the anti-adipogenesis effect of I-SLIM by oil red O staining and measuring the free triglyceride level.
MATERIALS AND METHODS
Sample Preparation: 10 mg of I-SLIM dissolved in dimethyl sulfoxide (DMSO) was taken for studies separately. DMEM high glucose along with 2% inactivated fetal bovine serum (FBS) is used to make up the volume to obtain a 1 mg/mL concentration stock solution and sterilized by 0.22 μ syringe filtration. For further studies, two-fold serial dilutions were prepared from the above mixture.
Cell Culture
National Centre for Cell Science, Pune, India, provided the 3T3-L1 (Mouse embryo Fibroblast) cell line. 10% inactivated FBS with DMEM high glucose were used to culture the stock cells, amphotericin B (5 µg/mL), penicillin (100 IU/mL), and streptomycin (100 µg/mL) in a humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with TPVG solution (0.2% trypsin, 0.02% ethylenediaminetetraacetic acid, and 0.05% glucose in phosphate-buffered saline [PBS]). The stock cultures were grown in 25 cm2 culture flasks, and all experiments were carried out in 96 mL plates (Tarsons India Pvt. Ltd., Kolkata, India).
Cytotoxicity Studies
The monolayer cell culture was trypsinized, and the cell count was adjusted to 100,000 cells/ml using DMEM high glucose containing 10% FBS. To each well of the 96 well microtiter plate, 0.1 mL of the diluted cell suspension was added. After 24 h, when a partial monolayer was formed, the supernatant was flicked off, washed the monolayer once with medium, and 100 µL of different test concentrations of test drugs were added on to the partial monolayer in microtiter plates. The plates were then incubated at 37°C for 3 days in 5% CO2 atmosphere, and microscopic examination was carried out, and observations were noted every 24 h interval. After 72 h, the drug solutions in the wells were discarded, and 50 µL of MTT in PBS was added to each well. The plates were gently shaken and incubated for 3 h at 37°C in 5% CO2 atmosphere. The supernatant was removed, and 100 µL of DMSO was added, and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 540 nm. The percentage growth inhibition was calculated using the following formula and concentration of test drug needed to inhibit cell growth by 50% (CTC50) values is generated from the dose-response curves for each cell line.
Anti-adipogenesis Assay
3T3-L1 cell culture and differentiation
To induce differentiation of cells, the 3T3-L1 cells were cultured in 4.5 g/L glucose-DMEM with 10% calf serum, antibiotic solution in 30 mm plastic Petri dishes until they reached 100% confluence. After 2 days, the 2-day post-confluent cells were incubated for 48 h in DMEM with 10% FBS, antibiotics, and a differentiation cocktail termed MDI, which contained 0.5 mM isobutylmethylxanthine, 1 µM dexamethasone, and 100 nm insulin. After 48 h, cells were maintained in DMEM with 10% FBS, insulin antibiotics for 6 days. After 6 days, the cells were maintained in DMEM with 10% FBS along with test samples (I-SLIM) for 24 h to investigate its anti-adipogenic activity.
Oil red O Staining
To determine the inhibitory effect of I-SLIM on lipid accumulation in 3T3-L1 cells, we used oil red O staining after adipocyte differentiation. The cells were rinsed with PBS and fixed with 10% formalin for 30 min. Then, the formaldehyde was removed, followed by washing with 60% isopropanol. After washing, the cells were stained for 1 h at room temperature in freshly diluted oil red O containing 0.5% oil red O in isopropanol. Finally, the dye retained in the 3T3-L1 cells was eluted with isopropanol and quantified by measuring the optical absorbance at 500 nm.
Triglyceride Assay
The cells were incubated with I-SLIM during adipogenesis. After differentiation, the cells were washed with PBS and lysed with lysis buffer, and the supernatants were collected and assayed for the triglyceride concentrations at 580 nm.
RESULTS
Cytotoxicity Effect of I-SLIM in 3T3-L1 Cell Line
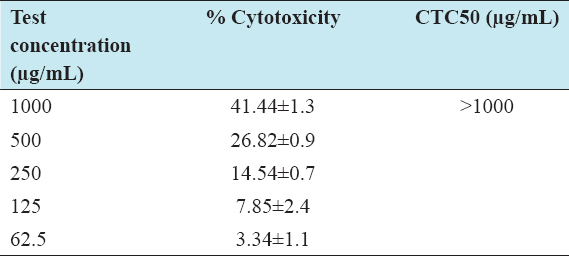
The cytotoxic effect of I-SLIM was determined in 3T3-L1 cell lines, with different concentrations (62.5, 125, 200, 500, and 1000 µg/mL) of I-SLIM for every 24 h interval till 72 h. According to the results, I-SLIM did not show any cytotoxic effect up to a concentration of 1000 µg/mL, even when the cells were treated for 72 h [Table 1].
Table 1: Cytotoxic properties of I-SILM against 3T3-L1 cell line
Anti-adipocyte Activity of I-SLIM in 3T3-L1 Cell Lines
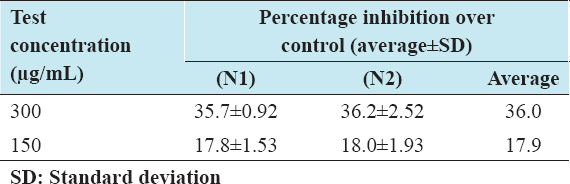
Once the cells were differentiated with two different concentrations (150 and 300 µg/mL) of I-SLIM, we used oil red O staining to know whether I-SLIM has an inhibitory effect on adipogenic differentiation. The result shows that I-SLIM inhibited lipid accumulation in 3T3-L1 dose-dependently compared with fully differentiated adipocytes. As shown in the quantitative results [Table 2], retained oil red O dye and intracellular triglyceride values were decreased in a dose-dependent manner [Table 3] when cells were treated with I-SLIM. These results demonstrated that I-SLIM reduced the accumulation of lipid in 3T3-L1 during adipogenic differentiation without cell cytotoxicity.
Table 2: In vitro anti-adipogenesis effect of I-SILM
Table 3: Level of released triglyceride in cell supernatant
DISCUSSION
Obesity has dramatically increased in most developing nations and is a prevalent condition related to metabolic disorders worldwide. Because the antiobesity drugs available at present are known to have various hazardous effects.[18] Hence, there is an immense importance for the natural produce having therapeutics property, as it is much safer compared to synthetic compounds.[19] The antiobesity ability of the phytochemicals is evaluated based on its potential to hinder the adipogenesis.[20]
Overweight and obesity are of major concern all over the world in many countries. It is more prevalent in developed countries, as well as in developing countries. For the patients with a body mass index range of 28–34, with metabolic complications, supplement therapy with weight management products is effective.[21]
I-SLIM regimen is a comprehensive lifestyle-based weight loss program which involves a synergy between herbal actives and nutrition, and thus, can initiate and maintain weight loss. I-SLIM supports overall holistic management of overweight and obesity.
Nowadays, investigation of the beneficial effects of several natural compounds in the adipocyte-related bioprocess is a new strategy of antiobesity research.[22] As shown in the oil red O staining and triglyceride assay results, I-SLIM decreased the lipid accumulation on the treated adipocytes compared with mature adipocytes.
CONCLUSION
The present study has focused on the inhibition of the formation of the fatty substances characteristic of adipogenesis, through the use of an herbal blend (I-SLIM), in conclusion, this study demonstrated a potent anti-adipogenic effect of herbal blend (I-SLIM) on cellular levels, implicating a potential application of this cost-effective natural product in the prevention of obesity.